Location Of Electron In Atom
Atoms and Light Energy
The written report of atoms and their characteristics overlap several different sciences. Chemists, Physicists, and Astronomers all must empathise the microscopic scale at which much of the Universe functions in lodge to run into the "bigger picture".
Inside the Atom
Simply like bricks are the building blocks of a dwelling, atoms are the building blocks of matter. Matter is anything that has mass and takes upward space (book). All matter is made up of atoms. The cantlet has a nucleus, which contains particles of positive charge (protons) and particles of neutral accuse (neutrons). Surrounding the nucleus of an atom are shells of electrons - minor negatively charged particles. These shells are actually different energy levels and within the free energy levels, the electrons orbit the nucleus of the atom.
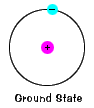 | The ground state of an electron, the energy level it normally occupies, is the country of everyman energy for that electron. |
| At that place is also a maximum energy that each electron tin have and all the same be role of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and information technology is considered to be ionized. | 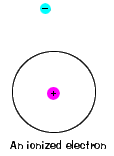 |
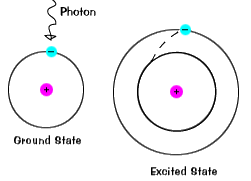 | When an electron temporarily occupies an energy land greater than its basis state, it is in an excited state. An electron can go excited if it is given actress energy, such as if it absorbs a photon, or packet of low-cal, or collides with a nearby atom or particle. |
Lite Energy
Each orbital has a specific free energy associated with information technology. For an electron to be boosted to an orbital with a college energy, it must overcome the deviation in energy between the orbital information technology is in, and the orbital to which it is going. This means that it must absorb a photon that contains precisely that corporeality of energy, or have exactly that amount of free energy from some other particle in a standoff.
The illustrations on this page are simplified versions of real atoms, of grade. Real atoms, even a relatively simple ones similar hydrogen, have many different orbitals, and so at that place are many possible energies with unlike initial and final states. When an cantlet is in an excited land, the electron can drop all the way to the footing state in ane get, or stop on the mode in an intermediate level.
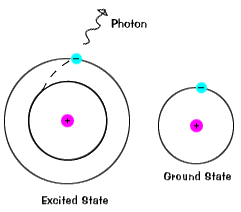 | Electrons practice not stay in excited states for very long - they presently return to their ground states, emitting a photon with the same energy equally the 1 that was absorbed. |
Identifying Individual Types of Atoms
Transitions amidst the various orbitals are unique for each chemical element because the energy levels are uniquely determined by the protons and neutrons in the nucleus. We know that different elements take dissimilar numbers of protons and neutrons in their nuclei. When the electrons of a sure cantlet return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of cantlet. This gives each element a unique fingerprint, making it possible to place the elements present in a container of gas, or even a star.
Nosotros can use tools similar the periodic table of elements to figure out exactly how many protons, and thus electrons, an cantlet has. First of all, we know that for an atom to take a neutral accuse, it must have the same number of protons and electrons. If an atom loses or gains electrons, information technology becomes ionized, or charged. The periodic table will give united states of america the atomic number of an chemical element. The atomic number tells united states of america how many protons an atom has. For example, hydrogen has an atomic number of 1 - which ways it has one proton, and thus i electron - and actually has no neutrons.
For the Student
Based on the previous description of the atom, draw a model of the hydrogen atom. The "standard" model of an atom is known as the Bohr model.Unlike forms of the same chemical element that differ merely by the number of neutrons in their nucleus are called isotopes. Most elements accept more than ane naturally occurring isotope. Many more isotopes have been produced in nuclear reactors and scientific laboratories. Isotopes usually aren't very stable, and they tend to undergo radioactive decay until something that is more stable is formed. Y'all may exist familiar with the element uranium - it has several unstable isotopes, U-235 being one of the nearly commonly known. The 235 ways that this form of uranium has 235 neutrons and protons combined. If we looked up uranium's atomic number, and substracted that from 235, we could calculate the number of neutrons that isotope has.
Hither'southward another example - carbon usually occurs in the form of C-12 (carbon-12) , that is, vi protons and 6 neutrons, though one isotope is C-13, with 6 protons and seven neutrons.
For the Student
Use the periodic tabular array and the names of the elements given below to effigy out how many protons, neutrons and electrons they have. Draw a model of an cantlet of the following chemical element: silicon-28, magnesium-24, sulphur-32, oxygen-16, and helium-4.For the Student
Using the text, define the post-obit terms: free energy levels, absorption, emission, excited country, footing country, ionization, atom, element, atomic mass, diminutive number, isotope.A Optional Note on the Breakthrough Mechanical Nature of Atoms
While the Bohr atom described above is a dainty way to larn well-nigh the structure of atoms, it is not the most authentic way to model them.
Although each orbital does take a precise energy, the electron is now envisioned equally being smeared out in an "electron cloud" surrounding the nucleus. It is common to speak of the mean distance to the cloud as the radius of the electron's orbit. So but retrieve, we'll keep the words "orbit" and "orbital", though we are now using them to draw not a apartment orbital plane, just a region where an electron has a probability of being.
Electrons are kept nearly the nucleus by the electric attraction between the nucleus and the electrons. Kept in that location in the aforementioned way that the ix planets stay virtually the Lord's day instead of roaming the milky way. Different the solar system, where all the planets' orbits are on the same aeroplane, electrons orbits are more 3-dimensional. Each energy level on an atom has a unlike shape. At that place are mathematical equations which volition tell you the probability of the electron'southward location within that orbit.
Let'southward consider the hydrogen atom, which nosotros already drew a Bohr model of.
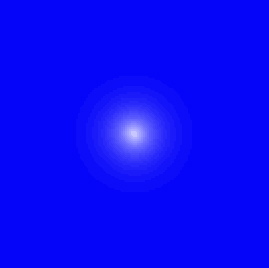 Probable locations of the electron in the footing state of the Hydrogen atom. | What you're looking at in these pictures are graphs of the probability of the electron's location. The nucleus is at the eye of each of these graphs, and where the graph is lightest is where the electron is almost likely to lie. What you see here is sort of a cantankerous section. That is, you accept to imagine the motion-picture show rotated around the vertical axis. So the region inhabited by this electron looks like a disk, just it should actually be a sphere. This graph is for an electron in its everyman possible energy state, or "ground land." |
| To the right is an excited state of hydrogen. Notice that at the center, where the nucleus is, the moving-picture show is nighttime, indicating that the electron is unlikely to be there. The two light regions, where the electron is most likely to exist institute, are really just one region. Remember, you take to mentally rotate this around a vertical axis, and then that in three dimensions the low-cal region is actually doughnut shaped. | 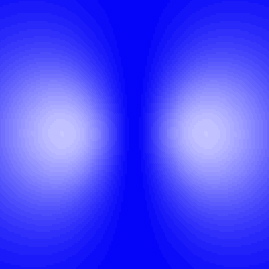 Probable locations of the electron in an excited state of Hydrogen. |
The text and images in this department were adapted from Dave Slaven's page on The Atom (see References below).
Reference URLs:
The Atom
http://webs.morningside.edu/slaven/Physics/cantlet/
Spectra
http://www.colorado.edu/physics/PhysicsInitiative/Physics2000/quantumzone/
The Periodic Table
http://www.webelements.com/
Back to the Principal Spectra Unit Menu
Location Of Electron In Atom,
Source: https://imagine.gsfc.nasa.gov/educators/lessons/xray_spectra/background-atoms.html
Posted by: cartwrightpospot.blogspot.com


0 Response to "Location Of Electron In Atom"
Post a Comment